Description
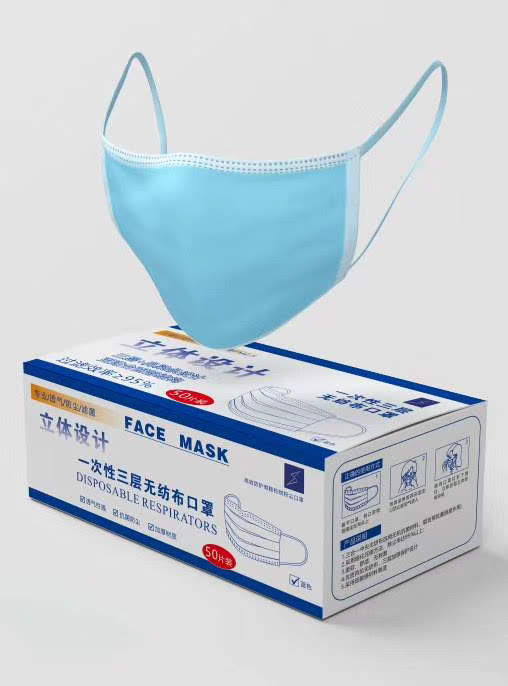
Surgical Masks Flat Face Masks Medical sterile mask
A surgical mask is a loose-fitting, disposable device that creates a physical barrier between the mouth and nose of the wearer and potential contaminants in the immediate environment. Surgical masks are regulated under 21 CFR 878.4040. Surgical masks are not to be shared and maybe labeled as surgical, isolation, dental, or medical procedure masks. They may come with or without a face shield. These are often referred to as face masks, although not all face masks are regulated as surgical masks.
Surgical masks are made in different thicknesses and with different abilities to protect you from contact with liquids. These properties may also affect how easily you can breathe through the face mask and how well the surgical mask protects you.
If worn properly, a surgical mask is meant to help block large-particle droplets, splashes, sprays, or splatter that may contain germs (viruses and bacteria), keeping it from reaching your mouth and nose. Surgical masks may also help reduce exposure of your saliva and respiratory secretions to others.
While a surgical mask may be effective in blocking splashes and large-particle droplets, a face mask, by design, does not filter or block very small particles in the air that may be transmitted by coughs, sneezes, or certain medical procedures. Surgical masks also do not provide complete protection from germs and other contaminants because of the loose fit between the surface of the face mask and your face.
Surgical masks are not intended to be used more than once. If your mask is damaged or soiled, or if breathing through the mask becomes difficult, you should remove the face mask, discard it safely, and replace it with a new one. To safely discard your mask, place it in a plastic bag and put it in the trash. Wash your hands after handling the used mask.
We offer Surgical Masks Flat Face Masks Medical sterile mask at wholesale price, contact us at info@www.plcskit.com for details.
All of these respirators have protection factors of at least 10 in the countries listed below, as outlined in the standards and guidance documents specified.
Use of respirators approved under standards used in other countries that are similar to NIOSH-approved N95 respirators
| Country | Performance Standard | Acceptable product classifications | Standards/Guidance Documents | Protection Factor ≥ 10 |
|---|---|---|---|---|
| Australia | AS/NZS 1716:2012 | P3
P2 |
AS/NZS 1715:2009 | YES |
| Brazil | ABNT/NBR 13698:2011 | PFF3
PFF2 |
Fundacentro CDU 614.894 | YES |
| China | GB 2626-2006 | KN 100 KP100
KN95 KP95 |
GB/T 18664—2002 | YES |
| Europe | EN 149-2001 | FFP3
FFP2 |
EN 529:2005 | YES |
| Japan | JMHLW-2000 | DS/DL3
DS/DL2 |
JIS T8150: 2006 | YES |
| Korea | KMOEL-2017-64 | Special
1st |
KOSHA GUIDE H-82-2015 | YES |
| Mexico | NOM-116-2009 | N100, P100, R100
N99, P99, R99 N95, P95, R95 |
NOM-116 | YES |
| US NIOSH Requirements | NIOSH approved 42 CFR 84 |
N100, P100, R100 N99, P99, R99 N95, P95, R95 |
OSHA 29CFR1910.134 | YES |